This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the global pharmaceutical inspection machines market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The global market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive, technical, market-oriented, and commercial study of the pharmaceutical inspection machines market. The secondary sources used for this study include the US Food and Drug Administration (FDA), European Medicines Agency (EMA), General Data Protection Regulation (GDPR), International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), UK Industrial Vision Association (UKIVA), Processing and Packaging Machinery Association (PPMA), Pharmaceutical Research and Manufacturers of America (PhRMA), Association for Packaging and Processing Technologies (PMMI), Association of Indian Medical Device Industry (AIMED), Japan Inspection Instruments Manufacturers’ Association (JIMA), National Institutes of Health (NIH), World Health Organization (WHO), Organisation for Economic Co-operation and Development (OECD), Eurostat Annual Reports, SEC Filings, Investor Presentations, Journals, Publications from Government Sources and Professional Associations, Interviews with Experts, and MarketsandMarkets Analysis, Company Websites, Annual Reports, SEC Filings, Investor Presentations, Press Releases, Expert Interviews, and MarketsandMarkets Analysis and among others. These sources were also used to obtain key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
Primary Research
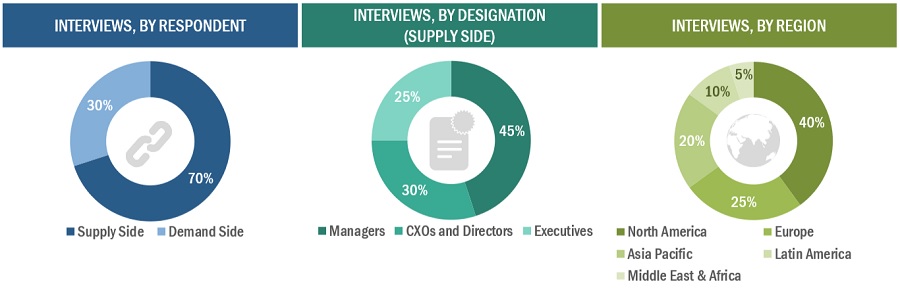
Extensive primary research was conducted after acquiring basic knowledge about the global pharmaceutical inspection machines market scenario through secondary research. Several primary interviews were conducted with market experts from the demand side, such as personnel from pharmaceutical and biotechnology industries, CMOs and CROs, and experts from the supply side, such as C-level and D-level executives, product managers, marketing & sales managers of key manufacturers, distributors, and channel partners. These interviews were conducted across four major regions, including the Asia Pacific, North America, Europe, Latin America, and the Middle East & Africa. Approximately 70% and 30% of the primary interviews were conducted with supply-side and demand-side participants, respectively. This primary data was collected through questionnaires, e-mails, online surveys, personal interviews, and telephonic interviews. The following is a breakdown of the primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
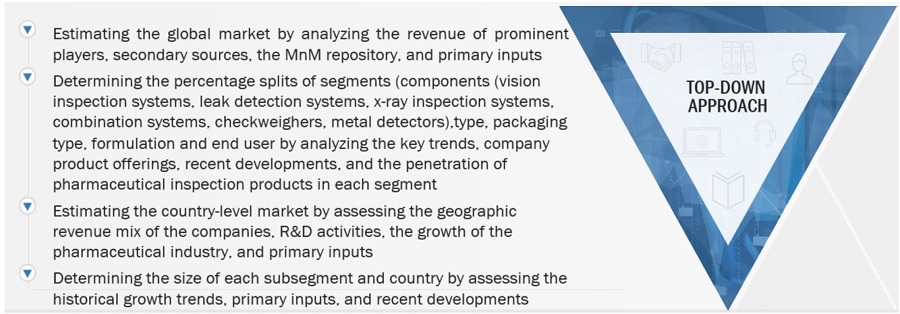
The global size of the market was estimated through multiple approaches. A detailed market estimation approach was followed to estimate and validate the value of the pharmaceutical inspection machines market and other dependent submarkets. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
-
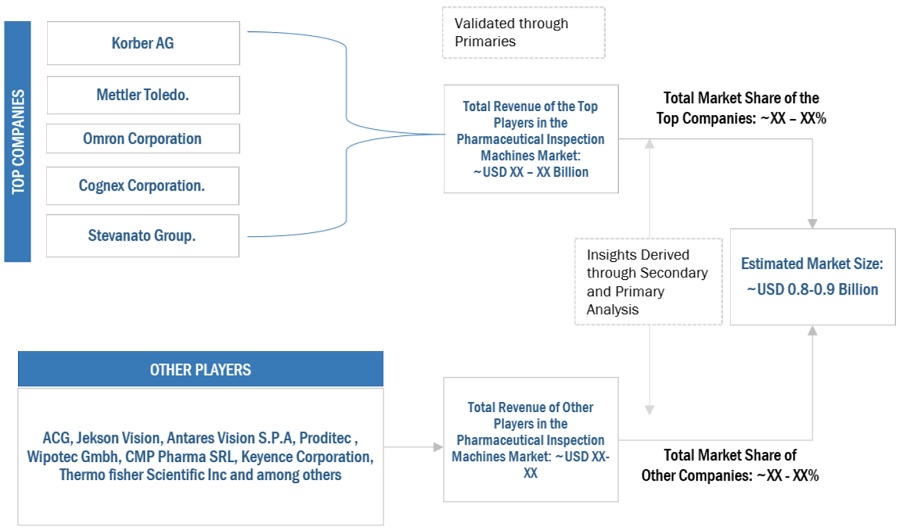
The key players in the industry and market have been identified through extensive primary and secondary research.
-
The revenues generated from the pharmaceutical inspection machines business of leading players have been determined through primary and secondary research.
-
All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources.
Market Size Estimation Methodology-Bottom-up approach
To know about the assumptions considered for the study, Request for Free Sample Report
Top-down Approach-
Data Triangulation
After estimating the overall market size from the market size estimation process, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply side.
Market Definition
Pharmaceutical inspection machines are crucial components of the quality control and assurance process. End users across the pharmaceutical industry extensively utilize these machines to ensure product quality and regulatory compliance with standards such as GMP. These machines can also seamlessly integrate into different systems. These machines are designed to inspect various types of pharmaceutical products, such as tablets, capsules, vials, ampoules, and syringes, for defects and contaminants.
Key Stakeholders
-
Inspection Machine Manufacturers
-
Inspection Machine Software Providers
-
Inspection Machine Distributors & Suppliers
-
Pharmaceutical & Biotechnology Companies
-
Government Associations
-
Market Research & Consulting Firms
-
Venture Capitalists & Investors
The main objectives of this study are as follows:
-
To define, describe, and forecast the global pharmaceutical inspection machines market based on the component, type, packaging type, formulation, end user, and region
-
To provide detailed information regarding the major factors influencing the growth of the market (such as drivers, restraints, and opportunities)
-
To strategically analyze micro markets with respect to individual growth trends, future prospects, and contributions to the overall pharmaceutical inspection machines market
-
To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
-
To forecast the size of the market segments with respect to five main regions, namely, North America, Europe, Asia Pacific, Latin America and the Middle East & Africa
-
To strategically profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies.
-
To track and analyze competitive developments such as acquisitions, product launches, expansions, agreements, partnerships, and R&D activities in the pharmaceutical inspection machines market.
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Geographical Analysis
-
Further breakdown of the Rest of Europe's pharmaceutical inspection machines market, by country
-
Further breakdown of the Rest of the Asia Pacific pharmaceutical inspection machines market, by country
Company Information
-
Detailed analysis and profiling of additional market players (up to five)



Growth opportunities and latent adjacency in Pharmaceutical Inspection Machines Market